The Solution for GRE Chemistry Practice Test [81-100]
추천글 : 【GRE】 Solution for GRE Chemistry Practice Test
101. Which of the following is a strong acid in pure liquid HF?

⑴ answer: B
102. What is the most common natural form in which fluorine is found on Earth?

⑴ answer: A
103. For a particle of mass m in a one-dimensional box of length L, the energy of the particle is given by the equation shown below. How much energy is required to promote the particle from the state with quantum number n = 2 to the state with quantum number n = 3?
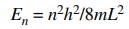

⑴ answer: B
⑵ ΔE = E3 - E2 = 9h2/8mL2 - 4h2/8mL2 = 5h2/8mL2
104. A large activation energy implies which of the following about a reaction?

⑴ answer: E
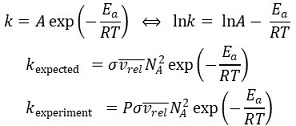
105. Analysis of a bottle of 100 mg vitamin C tablets yields an average vitamin C content of 99.8 mg, with a standard deviation of ±0.3 mg. Assuming Gaussian statistics, which of the following is true?
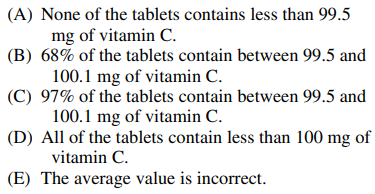
⑴ answer: B
⑵ mean ± 1 σ ⇔ 68% of the sample
106. In an experiment to test the de Broglie hypothesis, a beam of high-energy electrons with momenta
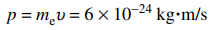
would be scattered by a nickel crystal with a pattern similar to that of which of the following?
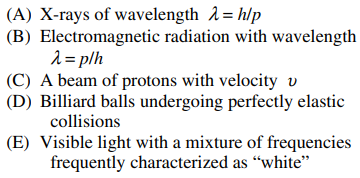
⑴ answer: A
⑵ It is not perfectly elastic collisons. See Compton scattering.
107. Which of the following is true about the quantum yield for photodecomposition of a chromophore?
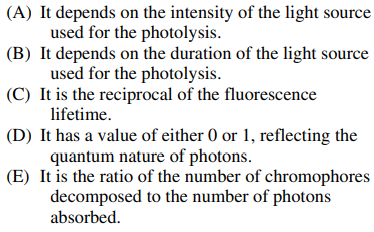
⑴ answer: E
⑵ Basically, the quantum yield means the effective number of photons devided by total number of photons.
108. Which of the following is the major organic product of the reaction sequence shown below?
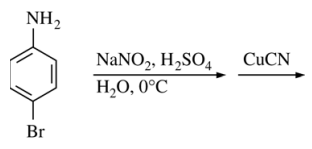
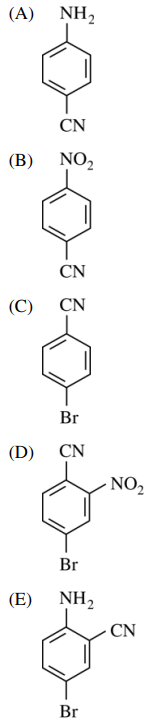
⑴ answer: C
⑵ diazotization by NaNO2 → Sandmeyer reaction
109. Which of the following is the major organic product of the sequence of reactions shown below?
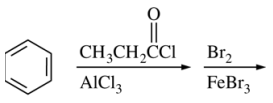
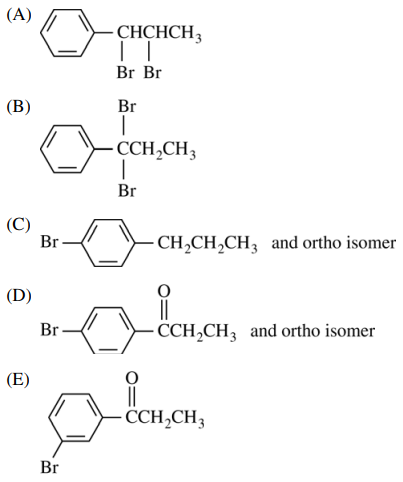
⑴ answer: E
⑵ step 1. Friedel-Craft acylation
⑶ step 2. EAS (bromination; para-substituted due to acyl group as a EWG)
110. A characteristic common to polymers that can be made to conduct electricity, such as polyacetylene and polypyrrole, is
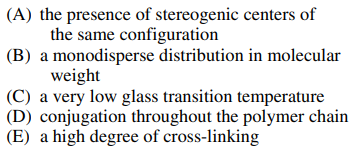
⑴ answer: D
111. Which of the following complexes does NOT contain a significant π component in the metal-ligand bonding?
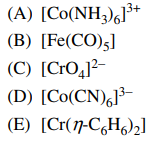
⑴ answer: A
⑵ For example, [Co(CN)6]3- has a bond of Co-C≡N ⇔ Co=C=N.
112. In an experiment to determine riboflavin by fluorescence spectrometry, a series of riboflavin standards was analyzed and gave a calibration line with a slope of 1000 ppm-1 and a y-intercept of 25. If a sample gave a fluorescence reading of 750, the riboflavin concentration (in ppm) of the sample is

⑴ answer: C
⑵ 750 = x × 1000 + 25 ⇔ x = 0.725
113. The rate constant for a first-order reaction R → P is 0.010 s-1. The concentration of R decreases to one-half of its initial value after
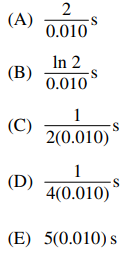
⑴ answer: B
⑵ first-order reaction : t1/2 = ln 2 / 0.010
114. The activated-complex theory (or transition state theory) assumes that an equilibrium exists between the

⑴ answer: A
115. Oxidation of (R)-3-bromo-5-hydroxypentanoic acid, shown below, yields the corresponding 3-bromopentanedicarboxylic acid product that is
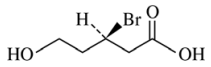

⑴ answer: E
⑵ There is a mirror surface at the middle of 3-bromopentanedicarboxylic acid.
116. Of the following molecules, which most readily undergoes a unimolecular elimination (E1) reaction?
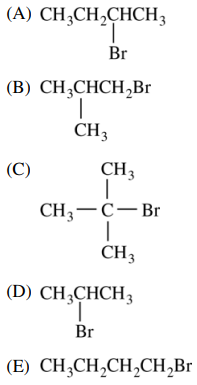
⑴ answer: C
⑵ The more substituted a given molecule is, the better it participates in E1 reaction.
117. Compounds have been prepared from which of the following noble gas elements?
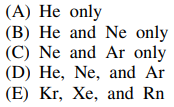
⑴ answer: E
118. AgCl is insoluble in water at room temperature. The dissolution of AgCl(s) into aqueous ammonia can best be explained as the
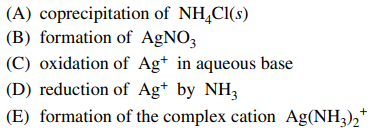
⑴ answer: E
119. The rate law shown below is for the reaction H2 + Br2 → 2HBr at the early stages of the reaction, when [HBr] is low and holds over a wide range of concentrations of H2 and Br2. An explanation that is consistent with the half-integer order in Br2 is given by which of the following?
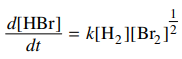
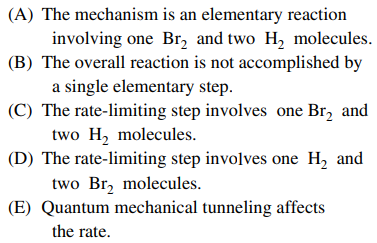
⑴ answer: B
120. In a mixture of He and Ar atoms in thermal equilibrium, what is the average speed of the He atoms, vHe, compared with the average speed of the Ar atoms, vAr? (mHe is the mass of He atoms, and mAr is the mass of Ar atoms.)
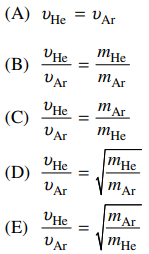
⑴ answer: E
⑵ v2 = 3RT / m
121. Which of the following is the major nucleophilic substitution product of the reaction shown below?
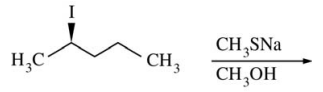
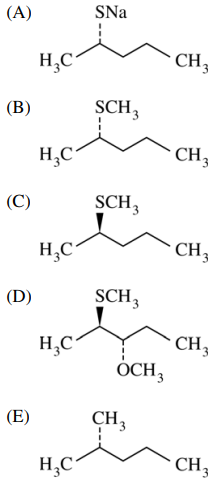
⑴ answer: B
122. What is the major organic product from the sequence of reactions shown below?
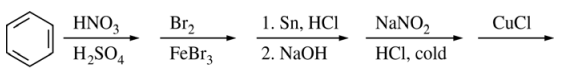
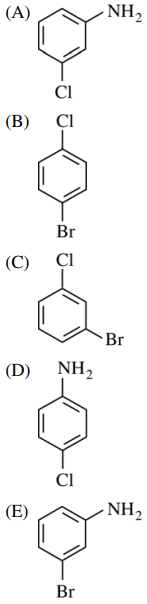
⑴ answer: C
⑶ step 1. HNO3, H2SO4 : nitration
⑷ step 2. Br2, FeBr3 : bromination (meta)
⑸ step 3. 1. Sn, HCl, 2. NaOH : NO2 → NH2
⑹ step 4. NaNO2, HCl, cold : diazotization
⑺ step 5. CuCl : Sandmeyer reaction
123. A simple electronic band structure for lithium metal is shown below. Based on this band structure, which of the following is correct?
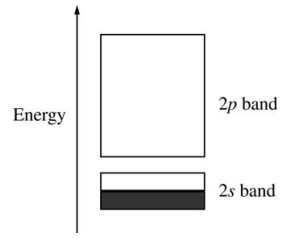

⑴ answer: E
⑵ Li = [He]2s1
124. Methyl t-butyl ether (MTBE), shown below, is a controversial gasoline additive. Of the following analytical techniques, which would be the best method to measure quantitatively trace amounts of MTBE in contaminated groundwater?
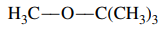

⑴ answer: B
125. For the species H2, HD, HT, and D2, all of the bond strengths (and force constants) are the same. Which of the following will have the lowest fundamental vibration frequency? (D = deuterium; T = tritium)

⑴ answer: D
⑵ In an analogy to pendulum, frequency is proportional to 1 / √(m1m2).
126. Which of the following is NOT true about Raman scattering?
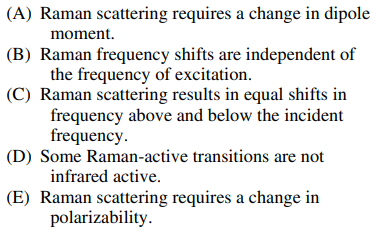
⑴ answer: A
127. Which of the following is formed when a solution of β-D-glucopyranose is allowed to stand in methanol that contains a small amount of an acid catalyst, as indicated in the equation shown below?
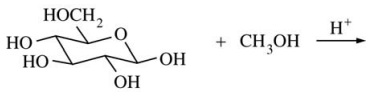
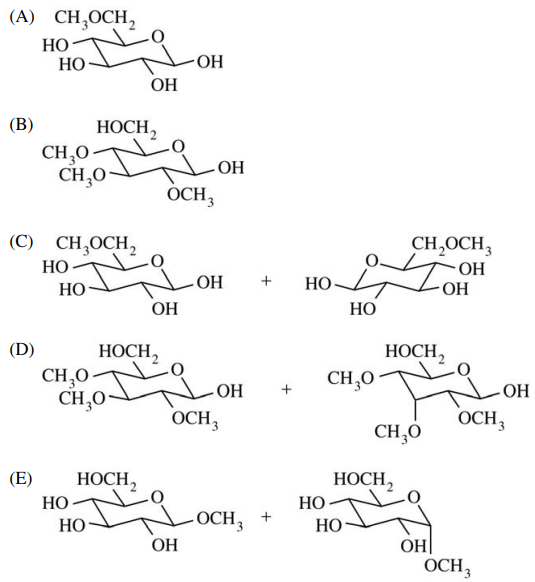
⑴ answer: E
⑵ step 1. The -OH next to the ether becomes -OHH+.
⑶ step 2. intramolecular SN2 reaction : -OH2 is removed.
⑷ step 3. Methanol approaches to the -C=O site and then forms -OCH3 (SN2-like, non-regioselective).
128. The species shown below is
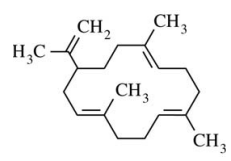

⑴ answer: C
129. Which of the following is NOT a known, relatively stable compound of uranium?
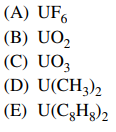
⑴ answer: D
130. The pKa1, pKa2, and pKa3 values for H3PO4 are given below. When 50.0 mL of 0.10 M Na2HPO4 are mixed with 50.0 mL of 0.10 M Na3PO4, the pH of the resulting solution will be closest to
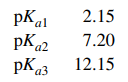

⑴ answer: E
⑵ Henderson-Hasselbalch equation : pH = pKa3 + log ([PO43-] / [HPO42-]) = pKa3 = 12.15
입력: 2023.06.18 15:42
'▶ 자연과학' 카테고리의 다른 글
| 【GR8677】 The Solution for GRE Physics Practice Test [21-40] (0) | 2023.07.29 |
|---|---|
| 【GR8677】 The Solution for GRE Physics Practice Test [01-20] (0) | 2023.07.29 |
| 【GR0627】 The Solution for GRE Chemistry Practice Test [81-100] (0) | 2023.06.18 |
| 【GR0627】 The Solution for GRE Chemistry Practice Test [61-80] (0) | 2023.06.12 |
| 【GR0627】 The Solution for GRE Chemistry Practice Test [41-60] (0) | 2023.06.11 |




최근댓글