The Solution for GRE Chemistry Practice Test [41-60]
추천글 : 【GRE】 Solution for GRE Chemistry Practice Test
41. The molecular geometry of IF5 is

⑴ answer: A

42. At a given temperature, the vapor pressure of SiF4 is significantly higher than that of SF4. The major physical basis for the difference in vapor pressure is that SiF4 and SF4 have different

⑴ answer: A
43. Which of the protons indicated will be observed as a doublet in the 1H NMR spectrum of the molecule shown below?

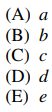
⑴ answer: B
⑶ a : singlet
⑷ b : doublet
⑸ c : triplet
⑹ d : quartet
⑺ e : triplet
44. Which of the following is the major product of the reaction shown below?
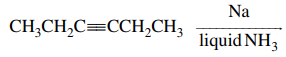

⑴ answer: D
45. Acyclic conjugated dienes may exist in two conformations, as shown below. Based on differences in steric strain, which of the following dienes has the greatest preference for the s-trans conformation?
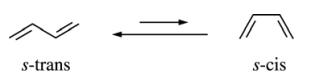
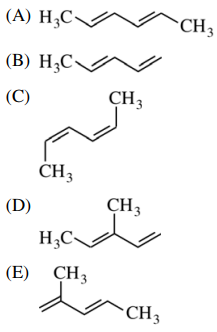
⑴ answer: C
46. Which of the following substances is in equilibrium with cyclopentanone and HCN shown below?
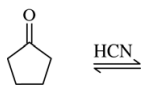
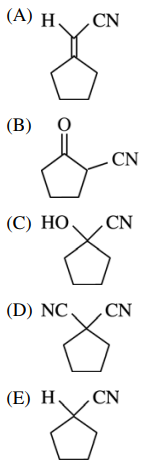
⑴ answer: C
47. All of the following elements have at least one isotope that is not radioactive EXCEPT
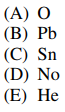
⑴ answer: D
48. Based on the molecular orbital model, which of the following is the number of unpaird electrons and the bond order for the superoxide ion, O2-?
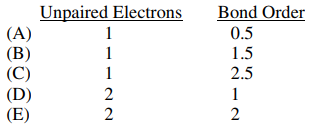
⑴ answer: B
⑵ See the electron configuration of O2.
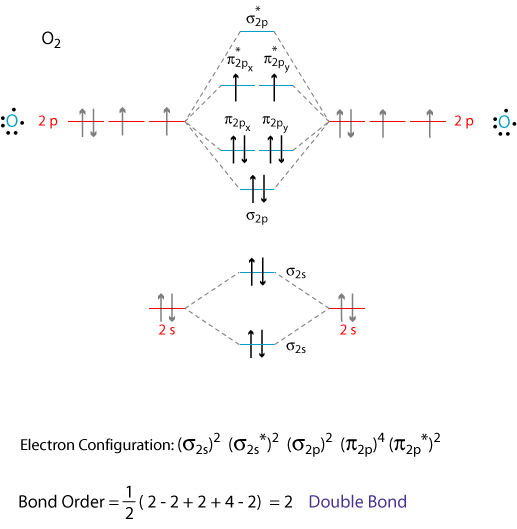
⑶ # of unpaired electron = 1 with an unpaired electron in either π2px or π2py.
⑷ bond order = (6 - 3) / 2 = 1.5
49. For a system at thermal equilibrium, which of the following is the Boltzmann distribution expression for the probability, pi, that a single molecule is in the ith energy state with energy εi?
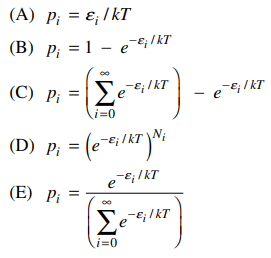
⑴ answer: E
⑵ Maxwell-Boltzmann speed distribution
50. Which of the following expressions involving fugacity, f, is correct as P → 0?
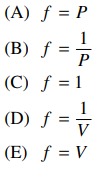
⑴ answer: A
51. Sodium acetate spontaneously crystallizes out of a supersaturated solution on standing or on the addition of a seed crystal. Which of the following is true for the thermodynamic quantities of this system for this process?
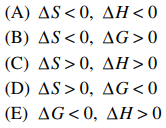
⑴ answer: A
⑵ spontaneous → ΔG < 0
⑶ crystallization → ΔS < 0
⑷ ΔG = ΔH - TΔS < 0 ⇔ ΔH < 0
52. If ideal gas behavior is assumed, for which of the following reactions does ΔH equal ΔU?
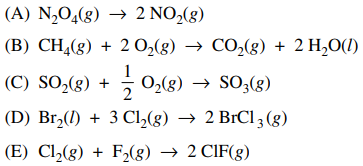
⑴ answer: E
⑵ ΔH = ΔU + Δ(PV) = ΔU + (Δn)RT = ΔU
⑶ ∴ Δngas = 0
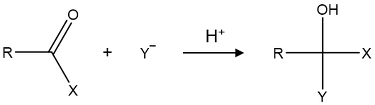
53. PbF2(s), which is slightly soluble in water, is dissolved in water to form a saturated solution in equilibrium with solid PbF2. Which of the following will cause additional PbF2(s) to dissolve?

⑴ answer: A
⑵ NO3- would decrease the amount of Pb2+ by forming Pb(NO3)2 (s).
54. Which of the following is the major product of the reaction shown below?
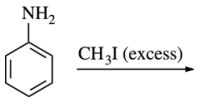
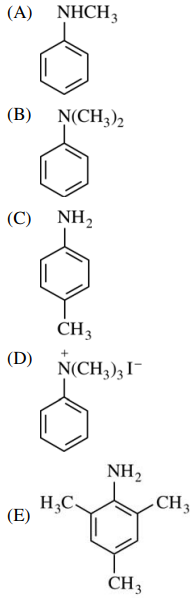
⑴ answer: D
⑵ SN2 substitution reaction by amine nucleophile
55. Which of the following is the product of the reaction shown below?
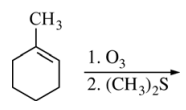
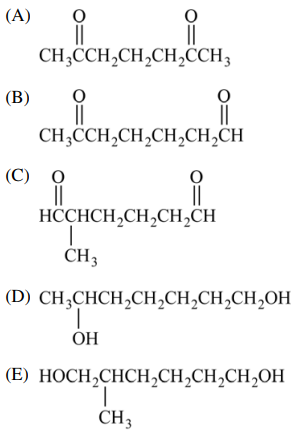
⑴ answer: B
⑵ alkene ozonolysis : Separate the double bonds and connect each with a carbonyl group (=O).
56. Which of the following is the major product of the reaction shown below?
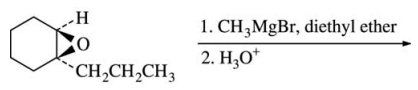
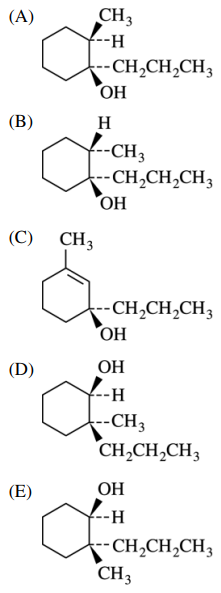
⑴ answer: B
⑵ Grigard reagend carbon-carbon coupling reaction for epoxide : The steric effect is considered.
57. Which of the following is the major organic product of the reaction shown below?
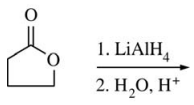
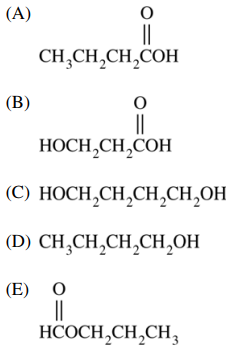
⑴ answer: C
⑵ saponification + carboxylic acid LAH reaction
58. Given the expression below, what is the value of (∂U / ∂V)T for an ideal gas undergoing isothermal expansion? (PV = nRT for an ideal gas.)
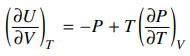
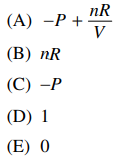
⑴ answer: E
⑵ The internal energy solely depends on the temperature.
59. The heat of fusion of ice is 333.5 J/g. The entropy change for the water when freezing 5.0 g of water at 0 ℃ and 1 atm pressure is

⑴ answer: E
⑵ ΔS = ΔQ / T = -333.5 J/g × 5.0 g / 273.15 K = -6.10 J/K
60. Many enzyme reactions follow the Michaelis-Menten rate law shown below, where V and Km are constants and [S] is the concentration of substrate that is undergoing a catalyzed reaction. When [S] ≫ Km, what is the apparent order of the reaction?
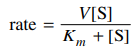

⑴ answer: A
⑵ It reaches the saturation velocity.
입력: 2023.06.11 10:49
'▶ 자연과학' 카테고리의 다른 글
| 【GR0627】 The Solution for GRE Chemistry Practice Test [81-100] (0) | 2023.06.18 |
|---|---|
| 【GR0627】 The Solution for GRE Chemistry Practice Test [61-80] (0) | 2023.06.12 |
| 【GR0627】 The Solution for GRE Chemistry Practice Test [21-40] (0) | 2023.06.06 |
| 【GR0627】 The Solution for GRE Chemistry Practice Test [01-20] (0) | 2023.06.04 |
| 【GR1724】 The Solution for GRE Biology Practice Test [180-194] (0) | 2023.05.30 |




최근댓글