The Solution for GRE Chemistry Practice Test [21-40]
추천글 : 【GRE】 Solution for GRE Chemistry Practice Test
21. Assuming complete dissociation, which of the following is NOT true about a 1.00 M Mg(NO3)2 solution? (Molar masses: Mg = 24.30 g; NO3- = 62.01 g; Mg(NO3)2 = 148.31 g)

⑴ answer: E
⑵ the amount of NO3- : 2 × 6.20 g = 12.40 g
22. A 499 mg sample of CuSO4·nH2O is heated to drive off the waters of hydration and then reweighed to give a final mass of 319 mg. Given that the sample contains 2.0 mmol of Cu, what is the average number of waters of hydration, n, in CuSO4·nH2O?

⑴ answer: B
⑵ H2O : (499 - 319) g ÷ 18 g/mol = 10 mol
⑶ ∴ n = 10 / 2.0 = 5.0
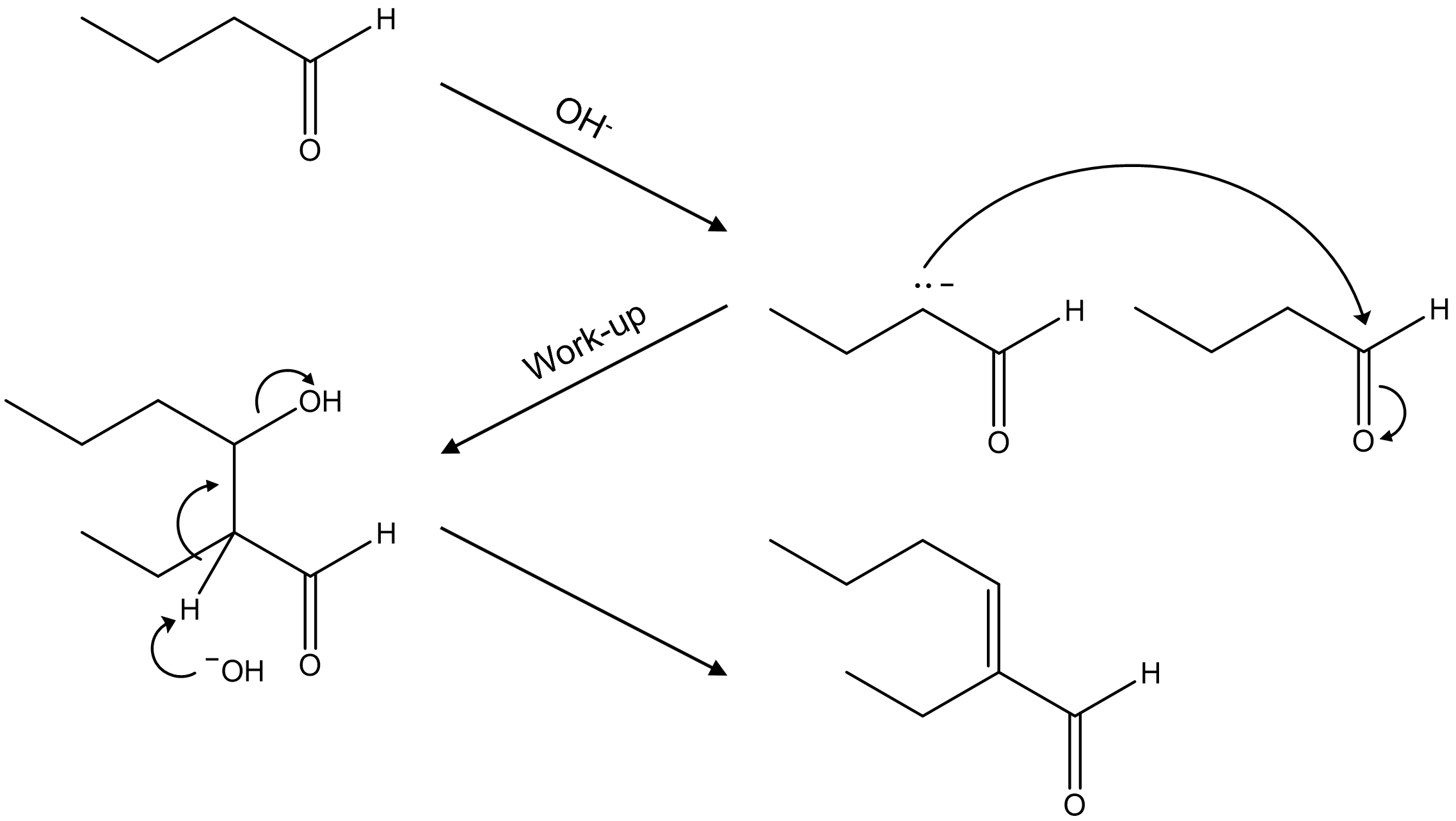
23. Which of the following is the aldol condensation product of butanal (CH3CH2CH2CHO)?

⑴ answer: E
24. Which of the following statements correctly applies Hückel's rule to the molecules shown below?
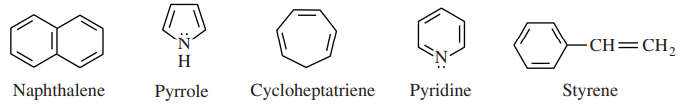

⑴ answer: C
25. When 1.0 kJ of heat is added to 5.0 L of an ideal gas, the gas expands against a constant external pressure of 1.0 bar to a final volume of 8.0 L. What is the change in internal energy, ΔU, for the gas? (1.0 L·bar = 0.10 kJ)

⑴ answer: B
⑵ q = 1.0 kJ = ΔH (∵ constant pressue) = ΔU + PΔV = ΔU + 1.0 × (8.0 - 5.0) × 0.10
⑶ ∴ ΔU = 0.70 kJ
26. Which of the following must be true for adiabatic processes?
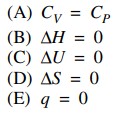
⑴ answer: E
27. At 37 ℃, the dissociation constant, Kw, of water is 2.5 × 10-14 (pKw = 13.6). What is the pH of a 1.0 × 10-5 M NaOH solution at 37 ℃?

⑴ answer: C
⑵ pOH = 5
⑶ ∴ pH = pKw - pOH = 13.6 - 5 = 8.6
28. The reaction shown below is not balanced. If the reaction is balanced using the smallest whole number coefficients possible, the coefficient for I- will be
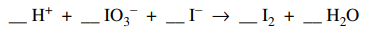
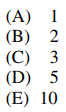
⑴ answer: D
⑵ oxidation number (ON)
⑶ IO3- (I : +5) → 0.5 I2 (I : 0), Δ ON = -5
⑷ I- (I : -1) → 0.5 I2 (I : 0), Δ ON = +1
⑸ ⑶ × 1 + ⑷ × 5, IO3- + 5I- → 3I2
⑹ complete rxn : IO3- + 5I- + 6H+ → 3I2 + 3H2O
29. Which of the following is the major product of the reaction shown below?
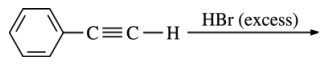
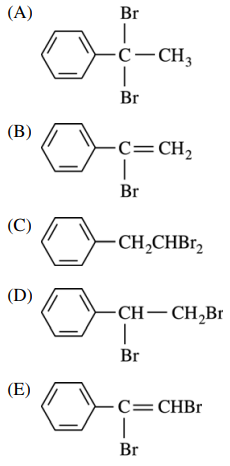
⑴ answer: A
⑵ Benzylic carbocation is highly stable compared to other carbocations.
30. The reaction of 2-bromobutane with methanol, as shown below, yields which of the following as the major product?
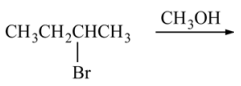
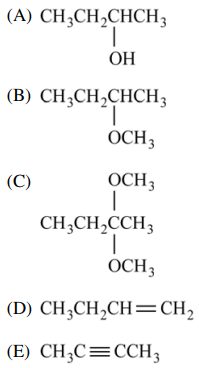
⑴ answer: B
31. Which of the following is the major organic product of the reaction shown below?
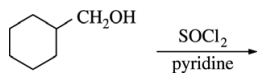
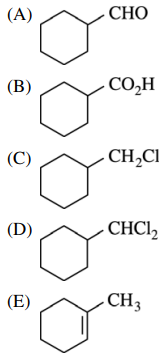
⑴ answer: C
32. In which of the following are the compounds shown below listed in order of increasing reactivity to acid-catalyzed dehydration?
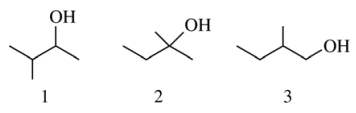
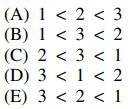
⑴ answer: D
⑵ 1 : The secondary carbocation is generated as an intermediate.
⑶ 2 : The tertiary carbocation is generated as an intermediate.
⑷ 3 : The primary carbocation is generated as an intermediate.
33. Two cylinders, one containing 1 mole of C4H10 gas at 1 atm and the other containing 1 mole of CH4 gas at 1 atm, are at 288 K. If each gas absorbs 100 J of heat under conditions of constant volume, which of the following is true?
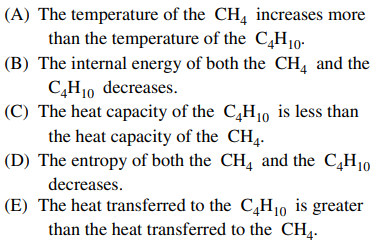
⑴ answer: A
⑵ ΔT = q / nCV = q / CV
⑶ CV for CH4 = 3/2 RT (cf. only translational movement component)
⑷ CV for C4H10 = (3/2 + 3/2 + 1/2) RT (cf. translational (3/2) + rotational (3/2) + vibrational (1/2))
34. Which of the following statement is true about a pure substance above its critical point?

⑴ answer: A
⑵ critical point : The maximum temperature at which liquid can be present.
⑶ triple point : Solid, liquid, and gas are in equilibrium.
35. If two wavefunctions Ψ1(x) and Ψ2(x) satisfy the condition given below, the two wavefunctions are
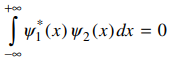

⑴ answer: A
36. For the equation ÂΨ = aΨ, where  and Ψ are shown be below, all of the following are true EXCEPT:


⑴ answer: E
37. At standard temperature and pressure, all of the following compounds exist in the gas state EXCEPT
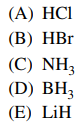
⑴ answer: E
⑵ LiH : white clear crystal lumps or powders
38. The electron configuration of Co in [Co(NH3)6]Cl3 is

⑴ answer: E
⑵ the oxidation number of Co = +3
⑶ the neutral state of Co = [Ar]4s23d7
⑷ the state of Co given = [Ar]3d6 (∵ 4s orbital electrons dissociate first, and 3d orbital electrons follow.)
39. A 0.600 g sample of a pure, weak diprotic acid gives end points at 20.0 mL and 40.0 mL when it is titrated with 0.100 M NaOH. What is the molar mass of the weak acid?

⑴ answer: D
⑵ 0.600 g ÷ x g/mol = 20.0 mL × 0.100 M = 2 mmol
⑶ ∴ x = 0.600 ÷ 0.002 = 300
40. The figure shown below is a plot of conductance data obtained during the titration of HCl with a standard solution of NaOH. Which of the following statements about the results is NOT true?
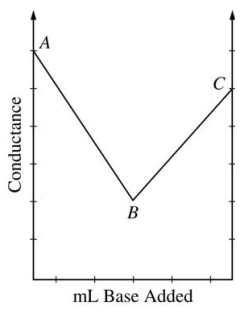
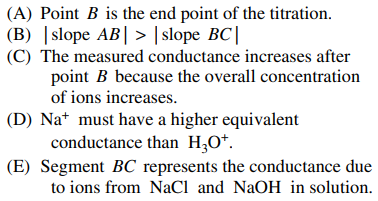
⑴ answer: D
⑵ conductance = k × ([H+] + [Cl-] + [Na+] + [OH-])
⑶ x = the amount of the standard solution added.
⑷ ([H+]0 + [Cl-]0) × V0 = ([Na+]0 + [OH-]0) × Veq
⑸ | slope AB | = k × ([H+]0 + [Cl-]0) × d/dx [1 - V0 / (V0+x) ]
∵ [H+] × V0 = ([H+] + [Na+]) × (V0+x) in AB)
⑹ | slope BC | = k × ([H+]0 + [Cl-]0) × d/dx [1 - V0 / (V0+x) ] + k × ([Na+]0 + [OH-]0) × d/dx [ (x - Veq) / (V0+x) ]
⑺ fact 1. | slope AB | and | slope BC | are not constant.
⑻ fact 2. | d/dx [1 / (1+x)] | decreases as x increases.
⑼ fact 3. At near to B, (x - Veq) ≪ Veq.
입력: 2023.06.05 01:38
'▶ 자연과학' 카테고리의 다른 글
| 【GR0627】 The Solution for GRE Chemistry Practice Test [61-80] (0) | 2023.06.12 |
|---|---|
| 【GR0627】 The Solution for GRE Chemistry Practice Test [41-60] (0) | 2023.06.11 |
| 【GR0627】 The Solution for GRE Chemistry Practice Test [01-20] (0) | 2023.06.04 |
| 【GR1724】 The Solution for GRE Biology Practice Test [180-194] (0) | 2023.05.30 |
| 【GR1724】 The Solution for GRE Biology Practice Test [160-179] (0) | 2023.05.29 |




최근댓글