The Solution for GRE Chemistry Practice Test [01-20]
추천글 : 【GRE】 Solution for GRE Chemistry Practice Test
1. Of the following, which element has the highest first ionization energy?

⑴ answer: A
⑵ first ionization energy : Rb < Sr < Ga < Ge < As

2. Which of the following is the most acceptable Lewis electron dot structure for carbon monoxide?

⑴ answer: C
⑵ Lewis electron dot structure determination
⑶ step 1. C:: :::O
⑷ step 2. :C=O:: → Not probable because octat rule is not established.
⑸ step 3. :C≡O: → 🄒
⑹ step 4. C≣O: → Not probable because octat rule is not established.
3. What is the correct IUPAC name for the compound shown below?
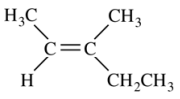
⑴ answer: C
⑵ step 1. E isomer (ref.)
⑶ step 2. main chain : 5 carbones. -pen-
⑷ step 3. functional group : alkene. -ene
⑸ step 4. numbering : The lower the alkene number, the more prioritized. Here, the alkene number is 2.
⑹ step 5. substituent : 3-methyl-
⑺ final nomenclature : (E)-3-methyl-2-pentene
4. What is the total number of stereoisomers possible for the compound shown below?
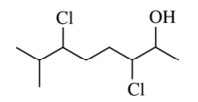
⑴ answer: D
⑵ Three chiral centers (i.e., carbons connected to -Cl or -OH) are distinct, so the number is 23 = 8.
5. The total number of peptide bonds in the structure shown below is
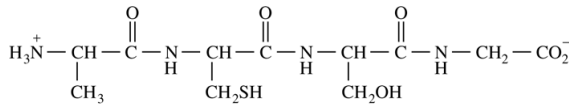
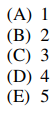
⑴ answer: C
⑵ # of peptide bonds = # of -CONH-
6. A 0.10 L solution of Cl- (aq) is titrated with 1.0 × 10-3 M Ag+ (aq). The end point is reached when 0.025 L of the Ag+ solution has been added. What was the concentration of Cl- in the original solution?

⑴ answer: B
⑵ x × 0.10 = 0.001 × 0.025 → x = 2.5 × 10-4 M
7. ΔH for the reaction shown below is greater than zero. Assuming ΔH is independent of temperature, which of the following statements about the percent yield of CO (g) is true?
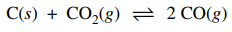

⑴ answer: B
⑵ principle of Le Chatelier : As the temperature of the system increases, the endothermic reaction prevails.
8. The initial rates given below were determined for the reaction A + 2B → AB2. What is the overall rate low for this reaction?

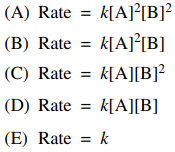
⑴ answer: D
⑵ 1st experiment ↔ 2nd experiment : rate ∝ [B]
⑶ 1st experiment ↔ 3rd experiment : rate ∝ [A]
⑷ ∴ rate = k[A][B]
9. Assuming that air is approximately 80 percent nitrogen and 20 percent oxygen by volume, which of the following is closest to the density of air at 0 ℃ and 1 atmosphere?

⑴ answer: C
⑵ At 0 ℃ and 1 atmosphere, the volume of 1 mol air is 22.4 L.
⑶ As the number of air particles is proportional to the volume percent, there are 80 % nitrogen and 20 % oxygen by number.
⑷ ∴ ρ = (0.8 mol × 28 g N2/mol + 0.2 mol × 32 g O2/mol) ÷ 22.4 L = 1.285714 g/L
10. How many π bonds are there in acetylene, shown below?
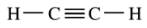
⑴ answer: B

11. In which of the following are the carboxylic acids shown below listed in order of decreasing acidity, from most acidic to least acidic?
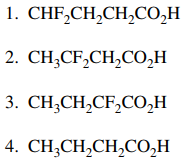
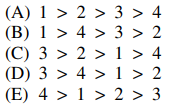
⑴ answer: C
⑵ inductive effect : σ bonding of electronegative atoms (e.g., F) increases acidity by withdrawing electrons.
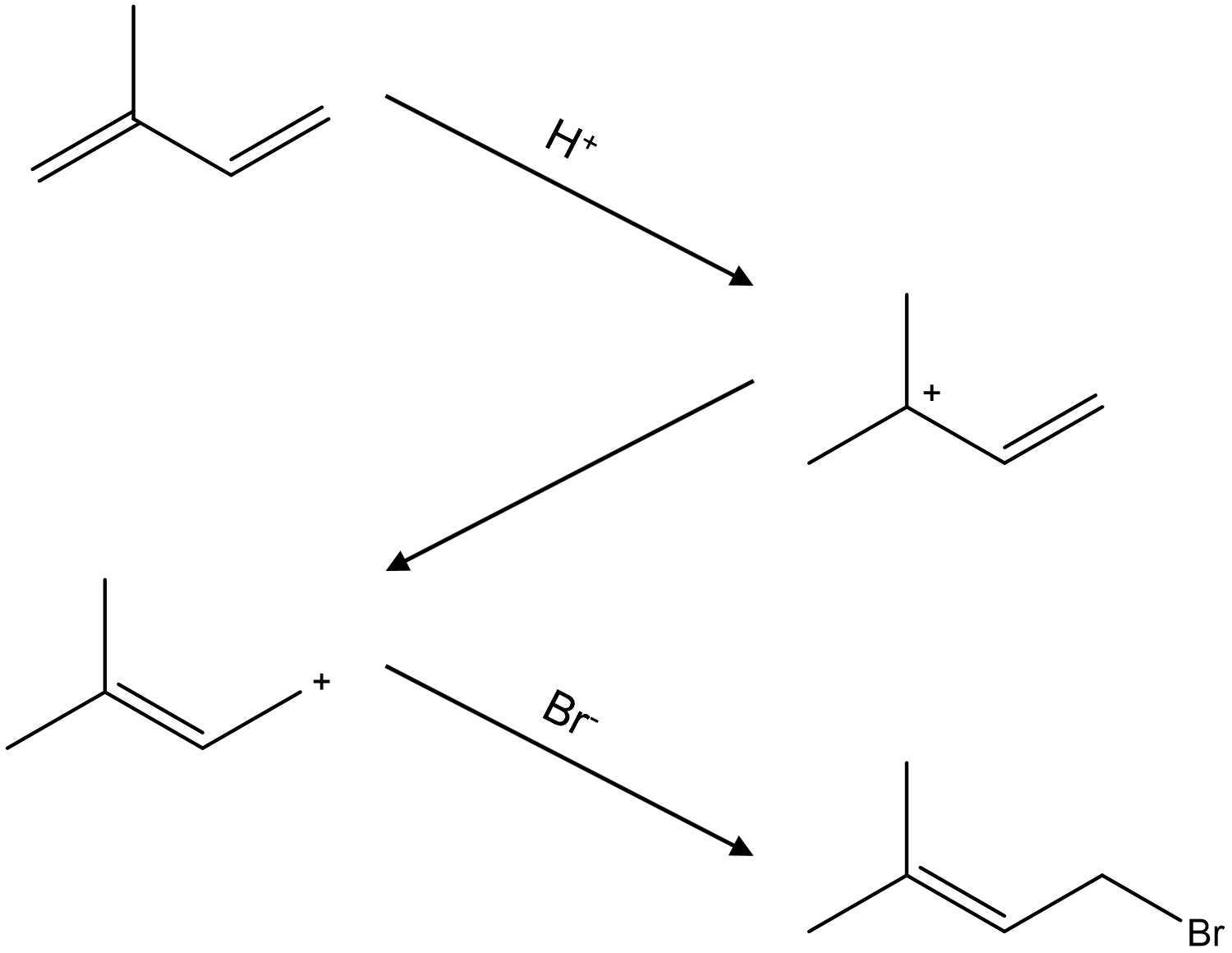
12. Which of the following is a 1,4-additional product of the reaction shown below?
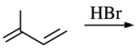
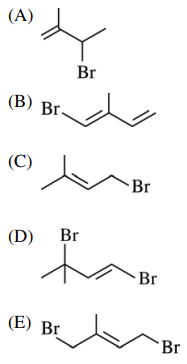
⑴ answer: C
⑵ hydrogen halide addition to alkene
13. Which of the following is a weak Brønsted–Lowry acid?
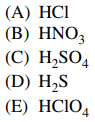
⑴ answer: D
⑵ acid : H+ donor
⑶ base : H+ acceptor
14. Which of the following correctly lists the species in order of increasing radius from smallest to largest?
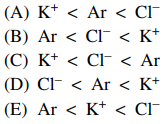
⑴ answer: A
⑵ When the number of electrons is the same, the radius decreases as the atomic number increases.
15. The half-life of 14C is 5,730 years. All of the following are true for the method of carbon dating EXCEPT:
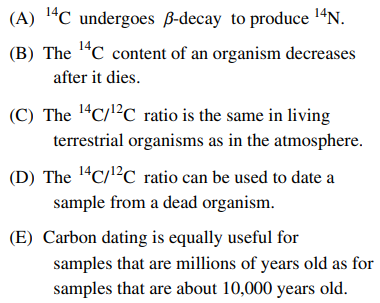
⑴ answer: E
⑵ β-decay
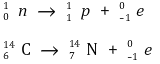
⑶ Samples that are millions of years old are too long to use carbon dating accurately.
16. The curve shown below illustrates the Pv̅ behavior of a real gas, where v̅ is the molar volume. According to the van der Waals model for nonideal gas behavior, the values of Pv̅ / RT greater than 1.0 at high pressures are due to
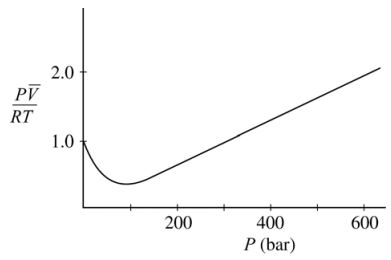

⑴ answer: C
⑵ van der Waals model
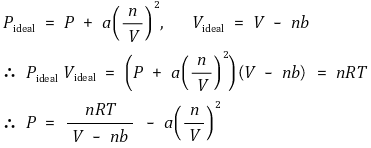
⑶ high pressure : The repulsive force by molecular interactions can be neglected.
⑷ high pressure : Rather, the volume occupied by the molecules should be considered due to the small total volume.
17. For the reaction shown below at 298 K, ΔGº = -163 kJ/mol. What is the value of the equilibrium constant, Kp, for this reaction?
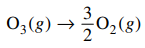
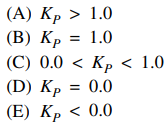
⑴ answer: A
⑵ ΔG = ΔGº + RT ln Kp = 0 ⇔ ΔGº = - RT ln Kp < 0
⑶ ∴ Kp > 1.0
18. In an isolated hydrogen atom, the 2px orbital has the same principal quantum number, n, as which of the following orbitals?
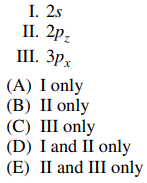
⑴ answer: D
19. Which of the following is NOT an allotrope of carbon?

⑴ answer: E
20. Of the following covalent bonds, which has the greatest bond dissociation energy?
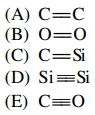
⑴ answer: E
⑵ Dissociation energy increases as the bond order increases, the atomic radius decreases, or the polarity (ΔEN) increases.
입력: 2023.06.05 01:38
'▶ 자연과학' 카테고리의 다른 글
| 【GR0627】 The Solution for GRE Chemistry Practice Test [41-60] (0) | 2023.06.11 |
|---|---|
| 【GR0627】 The Solution for GRE Chemistry Practice Test [21-40] (0) | 2023.06.06 |
| 【GR1724】 The Solution for GRE Biology Practice Test [180-194] (0) | 2023.05.30 |
| 【GR1724】 The Solution for GRE Biology Practice Test [160-179] (0) | 2023.05.29 |
| 【GR1724】 The Solution for GRE Biology Practice Test [141-159] (0) | 2023.05.21 |




최근댓글