The Solution for GRE Chemistry Practice Test [81-100]
추천글 : 【GRE】 Solution for GRE Chemistry Practice Test
81. Which of the following is a step in the mechanism of the hydrolysis of the ester shown below?


⑴ answer: A
82. Which of the following is the major product of the reaction shown below?

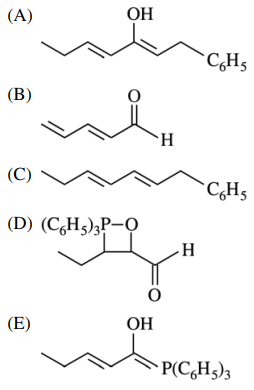
⑴ answer: C
83. For the reaction shown below, the experimental rate law is rate = k[A]2. Which of the following is the integrated rate law for this reaction?

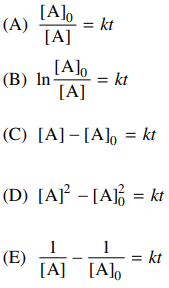
⑴ answer: E
84. Consider the mechanism shown below for oxidation of NO by O2. Based on the steady state approximation, which of the following conditions is true for this mechanism?
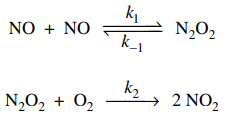
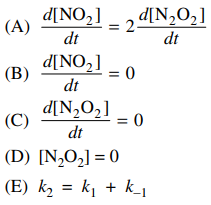
⑴ answer: C
⑵ semi-steady state assumption
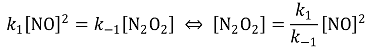
85. Given the information below, the concentrations of B and C and the control (thermodynamic or kinetic) of the system at short and long times are described by which of the following?
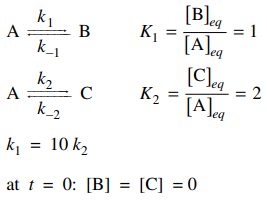
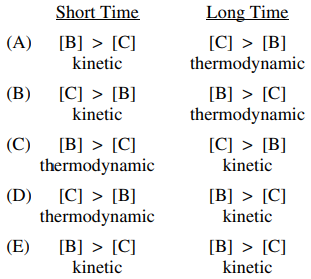
⑴ answer: A
86. In CrF2 (s), the coordination of the six F's around the Cr is a distorted octahedron with four short and two long Cr-F bonds. Which of the following best explains this observation?

⑴ answer: C
87. Each of the following molecules can act as a chelating ligand EXCEPT
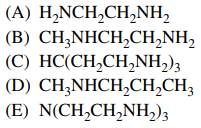
⑴ answer: D
⑵ In 🄐, 🄑, 🄒, and 🄔, a pair of Ns can provide lone pairs of electrons.
88. Which of the following is NOT a desirable property of an indicator to be used in a complexometric titration that involves EDTA?
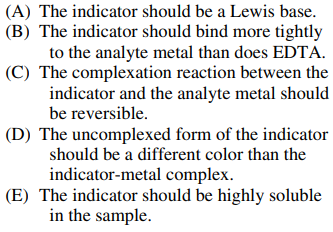
⑴ answer: B
89. Which of the following statements about complexes that form between metals, Mn+, and EDTA in aqueous solutions is true?
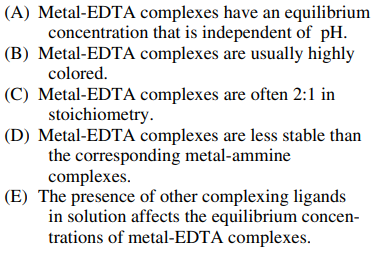
⑴ answer: E
⑵ quantification of metal cations using EDTA (MgY2-)
MgY2- + Mn+ → MYn-4 + Mg2+
⑶ 🄐 : MgY2- is denpendent on pH.
⑷ 🄑 : As metal-EDTA complexes are not colored, Eriochrome Black T or Calmagite is needed to quantify Mg2+.
⑸ 🄒 : Metal-EDTA complexes are often 1:1 in stoichiometry.
⑹ 🄓 : As metal-EDGA complexes are quite stable, the remaining Mg2+ can be credibly manipulated.
90. Which of the following compounds exists in stereoisomeric forms?
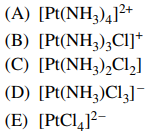
⑴ answer: C
⑵ cis/trans isomers
91. All of the following are recognized as pathways that can reduce the CO2 level in the atmosphere EXCEPT

⑴ answer: C
92. Which of the following is a wavefunction, ψ(r, θ, ϕ), for an s electron?
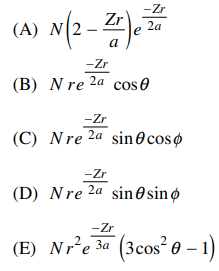
⑴ answer: A
⑵ s electron has symmetricity, independent of θ and ϕ.
93. Due to electron-electron interactions, it is not possible to obtain exact solutions to the Schrödinger equation for many-electron atoms. One approach that addresses this difficulty uses

⑴ answer: D
⑵ Slater's rule is adopted to address effective nuclear charges in the situation of many-electon atoms.
94. Of the following linear combinations of atomic orbitals centered on two atoms, A and B, which best represents the ground-state molecular orbital for the hydrogen molecule, H2?
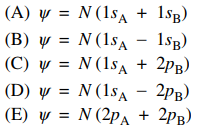
⑴ answer: A
⑵ N(1sA + 1sB) : bonding molecular orbital
⑶ N(1sA - 1sB) : anti-bonding molecular orbital
95. Acetic acid is extracted from ether into water. Which of the following actions will NOT increase the fraction of acetic acid removed from ether?

⑴ answer: D
⑵ 🄐 pH of the water increases. → Acetic acid becomes charged acetate ion. → Acetate ion is more acquired due to charge.
⑶ 🄑 Volume of water increases. → pH change of water due to acetic acid decreases. → Acetate ion is more acquired.
⑷ 🄒 Volume of ether decreases. → Acetic acid is more acquired due to diffusion.
⑸ 🄓 Benzoic acid is added. → Benzoic acid decreases pH of the water. → Acetate ion is less acquired.
⑹ 🄔 Ammonia is added. → Ammonia increases pH of the water. → Acetate ion is more acquired.
96. The ionic strength of an aqueous 0.10 M Pb(NO3)2 solution is

⑴ answer: C
⑵ NO3- does not make sediments in most cases.
97. Which two of the following are the propagation steps in the allylic bromination of cyclohexene shown below?
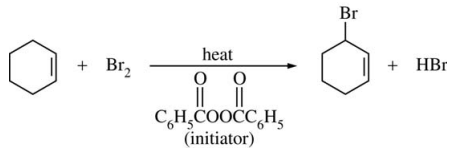
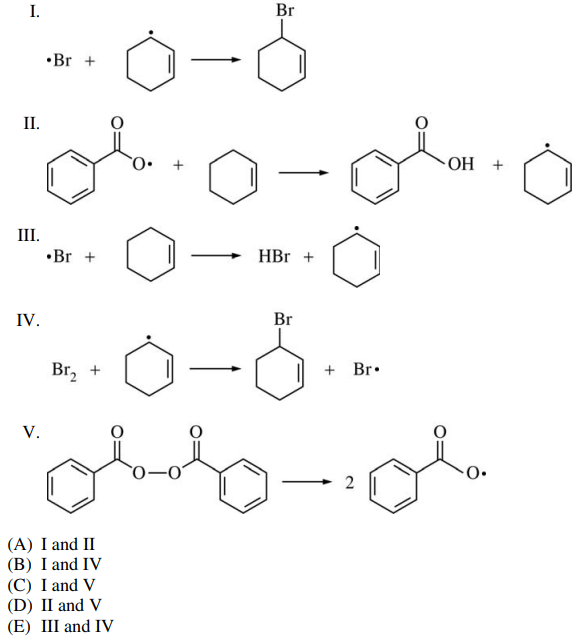
⑴ answer: E
⑵ X2 + Δ → radical substitution reaction
98. The transformation shown below is carried out by which of the following reagents?
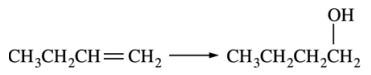
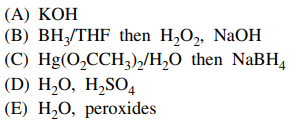
⑴ answer: B
⑵ anti-Markovnikov's rule in alkene hydration
99. Which of the following could carry out the conversion shown below?
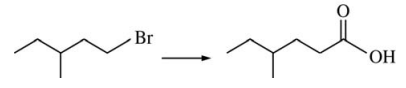
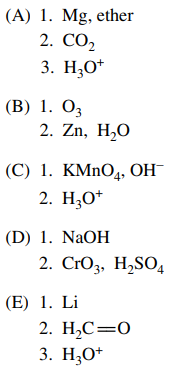
⑴ answer: A
⑵ Grignard reagent formation reaction → carbon-carbon coupling reaction
100. Vitamin B12, an essential nutrient for humans, contains which of the following elements?

⑴ answer: A
입력: 2023.06.18 13:11
'▶ 자연과학' 카테고리의 다른 글
| 【GR8677】 The Solution for GRE Physics Practice Test [01-20] (0) | 2023.07.29 |
|---|---|
| 【GR0627】 The Solution for GRE Chemistry Practice Test [101-130] (0) | 2023.06.18 |
| 【GR0627】 The Solution for GRE Chemistry Practice Test [61-80] (0) | 2023.06.12 |
| 【GR0627】 The Solution for GRE Chemistry Practice Test [41-60] (0) | 2023.06.11 |
| 【GR0627】 The Solution for GRE Chemistry Practice Test [21-40] (0) | 2023.06.06 |




최근댓글