The Solution for GRE Chemistry Practice Test [21-40]
추천글 : 【GRE】 Solution for GRE Chemistry Practice Test
21. Which of the following is always true of a spontaneous process?

⑴ answer: E
22. The equation ΔH = ΔU + PΔV is applicable

⑴ answer: B
⑵ ΔH = Δ(PV) = PΔV + VΔP, in general
23. A system that consists of a sample of nitrogen gas behaving as an ideal gas is compressed at a constant temperature. Which of the following is true about w (work) and q (heat transfer) for this process?

⑴ answer: A
⑵ chemistry: w > 0 when compressed, and w < 0 otherwise
⑶ physics: w < 0 when compressed, and w > 0 otherwise
⑷ based on u = q + w = constant, the sign of q can be determined
24. What is the maximum number of phases that can be at equilibrium with each other in a three-component mixture?
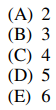
⑴ answer: D
⑵ Gibbs phase rule : P + F = C + 2
① P : number of phases
② F : degree of freedom, i.e. number of independent variables
③ C : number of components
④ Here, C = 3 and F = 0 (∵ equilibrium), so we get P = 5.
25. Infrared (IR) spectroscopy is useful for determining certain aspects of the structure of organic molecules because
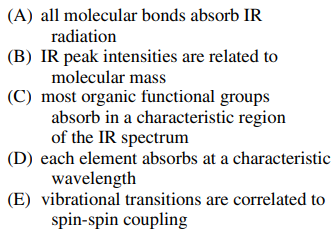
⑴ answer: C
26. Which of the following statements about nuclear binding energies is NOT true?
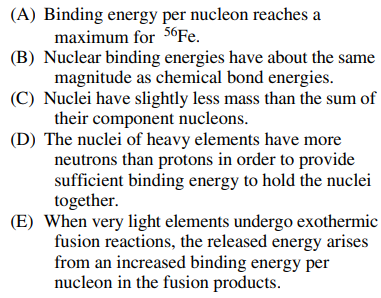
⑴ answer: B
⑵ The binding energy per nucleon is significantly stronger than the chemical binding energy to withstand the strong repulsion between protons.
27. The dissociation energy for a hydrogen-bromine bond is defined as the change in enthalpy, ΔH, for which of the following reaction?
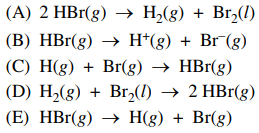
⑴ answer: E
⑵ The dissociation energy is based on the gaseous state.
28. A radioactive isotope, which is used in diagnostic imaging, has a half-life of 6.0 hours. If a quantity of this isotope has an activity of 150 μCi when it is delivered to a hospital, how much activity will remain 24 hours after delivery? (μCi = microcuries)

⑴ answer: D
⑵ I = I0 (1/2)t/T
29. The rate, r, of a zero-order chemical reaction A → B can be expressed as which of the following?
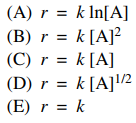
⑴ answer: E
30. Which of the following is classified as a conjugate acid-base pair?
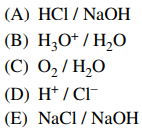
⑴ answer: B
⑵ At least, the major chemical species should be maintained when it comes to a conjugate acid-base pair.
31. An impure sample of K2O was analyzed by precipitating the potassium as the insoluble tetraphenyl borate salt, KB(C6H5)4. The precipitate, KB(C6H5)4 had a mass of 1.57 g. The mass of K2O in the original sample is obtained from which of the following? (Molar masses: KB(C6H5)4 = 358.3 g and K2O = 94.2 g)
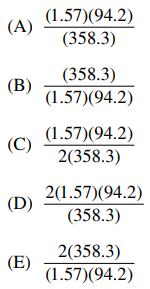
⑴ answer: C
⑵ x mg K2O × 1 mol K2O / 94.2 g × 2 mol K / mol K2O
⑶ 1.57 g KB(C6H5)4 × 1 mol KB(C6H5)4 / 358.3 g × 1 mol K / mol KB(C6H5)4
⑷ from ⑵ = ⑶, x = (1.57)(94.2) / 2(358.3)
32. Which of the following are the major products of the reaction shown below?
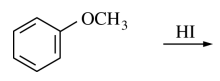
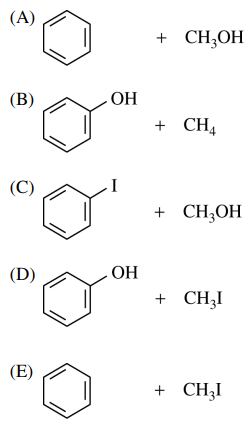
⑴ answer: D
⑵ step 1. Ph-OMe → Ph-O+(H)(Me)
⑶ step 2. SN2 reaction by a nucleophice I-
33. Of the following compounds, which has the fastest SN1 reaction rate with H2O in acetone?
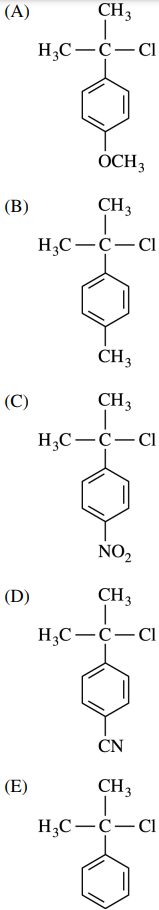
⑴ answer: A
⑵ Among the substituents, the strongest electron donating group (EDG) is -OMe.
34. Of the following, which compound is in equilibrium with the greatest percentage of its enol isomer?
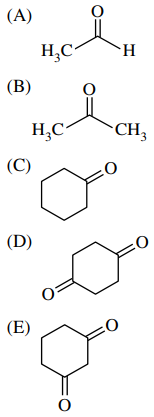
⑴ answer: E
⑵ If the enol is formed in compound (E), it is anticipated to generate additional resonance contributors from the enol form.
35. In which of the following are the molecules shown below listed in order of increasing reactivity toward electrophilic aromatic substitution?
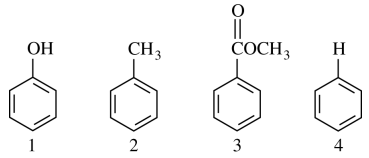
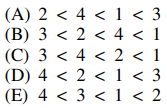
⑴ answer: C
⑵ The stonger electron donating group (EDG) will react EAS much eaiser.
36. Considering 0.1 M acqueous solutions of each of the following, which solution has the lowest pH?
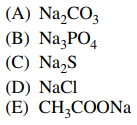
⑴ answer: D
⑵ Every other chemical species than NaCl are bases.
37. Of the following compounds, which has the lowest melting points?

⑴ answer: A
⑵ Because of the Van der Waals force, the larger the surface area, the higher the melting point.
38. Of the following solutions, whicih will have the highest ionic strength? (Assume complete dissociation.)

⑴ answer: A
⑵ Ionic strength increases as the average distance between ions decreases and the average absolute value of ions increases.
39. The KP for the reaction shown below is 0.26 at 1,000 ℃ and 40.8 at 1,300 ℃. Which of the following combinations of ΔH and ΔS are most plausible for this reaction at these temperatures?
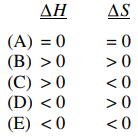
⑴ answer: B
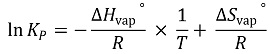
40. At a given temperature, the forward rate constant, k1, for the one-step reaction shown below is 4 × 10-7 M-1 s-1. Given that the equilibrium constant is 1 × 10-2, what is the reverse rate constant, k-1?
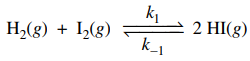
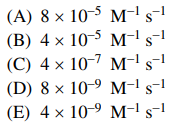
⑴ answer: B
⑵ K = 1 × 10-2 = k1 / k-1 → k-1 = 4 × 10-7 ÷ (1 × 10-2) = 4 × 10-5 M-1 s-1
입력: 2022.03.01 13:51
'▶ 자연과학' 카테고리의 다른 글
| 【GR1727】 The Solution for GRE Chemistry Practice Test [61-80] (0) | 2022.03.04 |
|---|---|
| 【GR1727】 The Solution for GRE Chemistry Practice Test [41-60] (0) | 2022.03.03 |
| 【GR1727】 The Solution for GRE Chemistry Practice Test [01-20] (0) | 2022.03.01 |
| 【닥터스톤】 닥터스톤의 과학기술 레시피 모음 (0) | 2022.02.20 |
| 【물리학】 물리2 포물선 운동 고난이도 문제 #3 (0) | 2020.05.23 |




최근댓글