The Solution for GRE Chemistry Practice Test [41-60]
추천글 : 【GRE】 Solution for GRE Chemistry Practice Test
41. Given the standard molar entropies listed below, the standard reaction entropy, ΔS°, in J K-1 mol-1, for the decomposition of calcium carbonate into calcium oxide and carbon dioxide is


⑴ answer: E
⑵ CaCO3 (s) → CaO(s) + CO2(g)
42. The Rydberg equation given below accurately predicts the UV-visible emission spectrum of the hydrogen atom. A form of the Rydberg equation may also be used to predict the UV-visible emission for all of the following EXCEPT


⑴ answer: A
⑵ Only species with a single electron can be applied to the equation.
43. Phtalic acid, (COOH)C6H4(COOH), is a weak, diprotic acid with dissociation constant below. The pH of an aqueous solution of potassium acid phthalate, (COOH)C6H4(COO-K+), is closest to


⑴ answer: D
⑵ ( pKa1 + pKa2 ) / 2
44. Which of the following is true for Br2 at standard temperature and pressure?

⑴ answer: B
⑵ color organic materials : Br2, FeCl3, AgNO3, KMnO4, Cr, NH4Cl, etc
45. On the basis of oxidation-reduction potential, which of the following is most likely to occur?
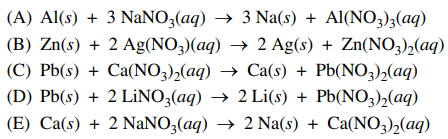
⑴ answer: B
⑵ ionization tendency: K>Ca>Na>Mg>Al>Zn>Fe>Ni>Sn>Pb>H>Cu>Hg>Ag>Pt>Au
⑶ In (E), Ca(s) will react with H2O.
46. Cobalt-60 is used in the radiation therapy of cancer and can be produced by bombardment of cobalt-59 with which of the following?

⑴ answer: A
47. Which of the following are the products of the reaction shown below?
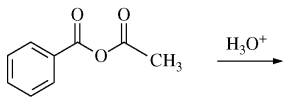
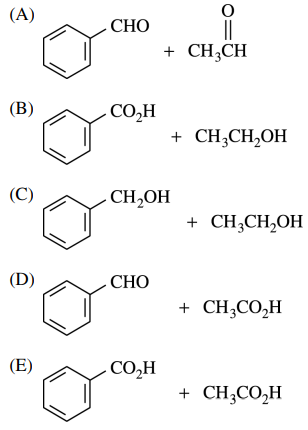
⑴ answer: E
⑵ acid-catalyzed anhydride hydration reaction
48. What is the product of the reaction shown below for para-cresol?
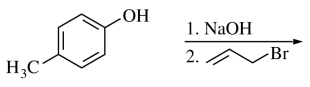
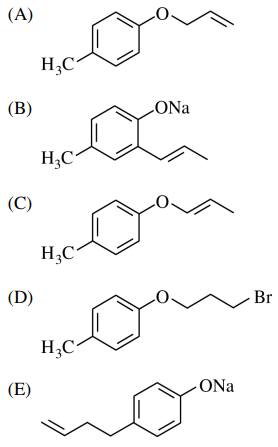
⑴ answer: A
⑵ step 1. Me-Ph-OH → Me-Ph-O-Na+
⑶ step 2. alkyl halide SN2 reaction
49. At 25 ℃, the maximum amound of PbI2 that can be dissolved in 1.00 L of pure water is 1.0 mmol. Assuming complete dissociation, the solubility product, Ksp, for lead iodide at 25 ℃ is
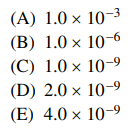
⑴ answer: E
⑵ Ksp = [ Pb2+ ] × [ I- ]2 = 10-3 × (2 × 10-3)2 = 4 × 10-9
50. Which of the following must be true if the wavefunction ψ(x) is normalized?
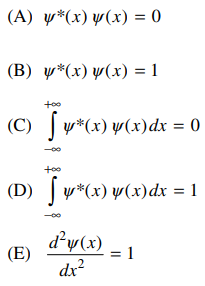
⑴ answer: D
51. If ψ(r) is the wavefunction for a 1s electron, the average distance from the nucleus for the electron is equal to
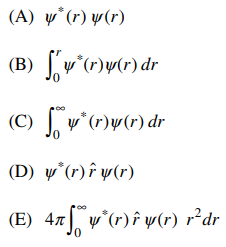
⑴ answer: E
52. Which of the following experimental observations were explained by Planck's quantum theory?

⑴ answer: A
53. The +1 oxidation state is more stable than the +3 oxidation state for which group 13 element?
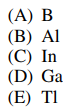
⑴ answer: E
⑵ It is due to f orbital.
54. The anhydride of Ba(OH)2 is
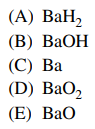
⑴ answer: E
⑵ Ba(OH)2 - H2O = BaO
55. A 0.217 g sample of HgO (molar mass = 217 g) reacts with excess iodide ions according to the reaction shown below. Titration of the resulting solution requires how many mL of 0.10 M HCl to reach equivalence point?
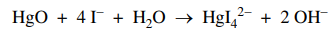

⑴ answer: C
⑵ 0.217 g ÷ 217 g / mol × 2 = 0.10 M × x mL × 1L / 1000 mL → x = 20 mL
56. The Hamiltonian operator for a particle in a one-dimensional box, whose potential is zero inside the box and infinite outside the box, is
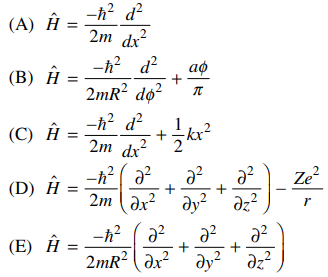
⑴ answer: A
⑵ Hamiltonian operator : It represents energy terms.
⑶ condition 1. one-dimensional : 🄓 and 🄔 are inappropriate.
⑷ condition 2. Schrödinger equation : 🄐 is chosen.
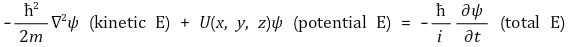
57. The normal modes of a carbon dioxide molecule that are infrared-active include which of the following?
Ⅰ. Bending
Ⅱ. Symmetric stretching
Ⅲ. Asymmetric stretching
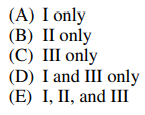
⑴ answer: D
58. Which of the following types of spectroscopy is a light-scattering technique?

⑴ answer: C
59. When a certain metal is irradiated with radiation of frequency 5.5 × 1014 s-1, electrons are ejected. If the work function of the metal is 2.9 × 10-19 J, which of the following expresses the kinetic energy (in joules) of the ejected electrons?
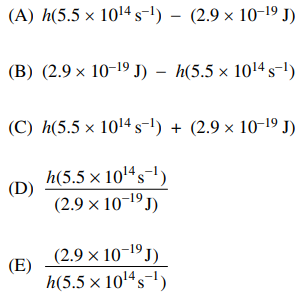
⑴ answer: A
60. The product of the reaction shown below is produced via which of the following intermediates?
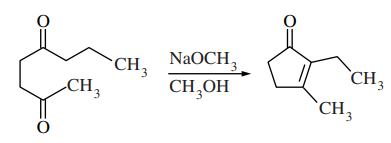
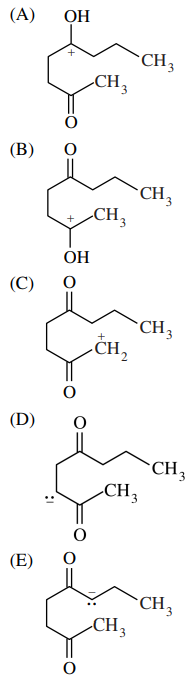
⑴ answer: E
⑵ intramolecular aldol reaction
입력: 2022.03.03 23:35
'▶ 자연과학' 카테고리의 다른 글
| 【GR1727】 The Solution for GRE Chemistry Practice Test [81-100] (0) | 2022.03.05 |
|---|---|
| 【GR1727】 The Solution for GRE Chemistry Practice Test [61-80] (0) | 2022.03.04 |
| 【GR1727】 The Solution for GRE Chemistry Practice Test [21-40] (0) | 2022.03.01 |
| 【GR1727】 The Solution for GRE Chemistry Practice Test [01-20] (0) | 2022.03.01 |
| 【닥터스톤】 닥터스톤의 과학기술 레시피 모음 (0) | 2022.02.20 |




최근댓글