The Solution for GRE Chemistry Practice Test [81-100]
추천글 : 【GRE】 Solution for GRE Chemistry Practice Test
81. Which of the following starting materials could be used in a Diels-Alder reaction to prepare the bicyclic product shown below?


⑴ answer: B
⑵ Diels-Alder reaction normally follows endo-orientation with 4+2 cyclic addition.
82. Which of the following is the major organic product of the reaction shown below?


⑴ answer: A
⑵ hydroboration-oxidation reaction of alkene
83. Which of the following procedures gives the compound shown below?
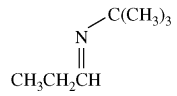
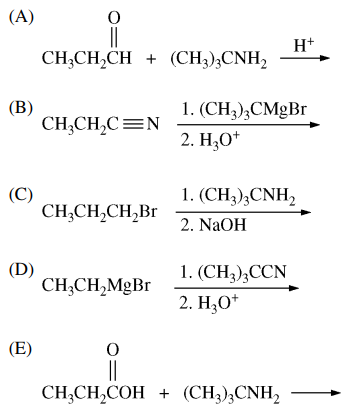
⑴ answer: A
⑵ amind addition reaction of aldehyde
84. Ionizing radiation can be detected using gas-filled tubes in which released electrons migrate to a collector electrode, producing a pulse. On the figure shown below, which region would give the largest detector response per incident photon?
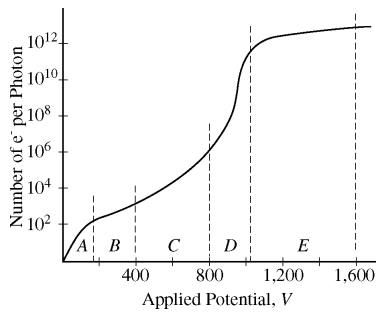
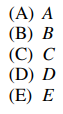
⑴ answer: E
⑵ The response of detector is dependent on the number of photons, thus the applied potential should be large.
85. Which of the following is required for both paramagnetism and ferromagnetism?

⑴ answer: E
⑵ According to the molecular orbital theory, the existence of unpaired electrons can make magnetism.
86. Redox enzyme catalysis involves the cyclic oxidation and reduction of metal ions that have at least two stable positive oxidation states. Which of the following groups of metals could be found at the active site of redox enzymes?
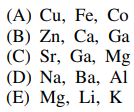
⑴ answer: A
⑵ Only chemical species with electrons in d orbital have various oxidation states.
⑶ Elements with less atomic number than Sc don't have electrons in d orbital.
87. The solid-state structures of the principal allotropes of elemental boron are made up of which of the following structural units?

⑴ answer: A
88. All proteins absorb electromagnetic radiation of wavelength around 190 nm, which corresponds to a π → π* excitation in the protein molecule. In which region of the spectrum is this wavelength found?

⑴ anwser: B
⑵ UV light spans a range of wavelengths between about 10 and 400 nanometers.
⑶ π → π* excitation is the important principle of UV-Vis spectrum.
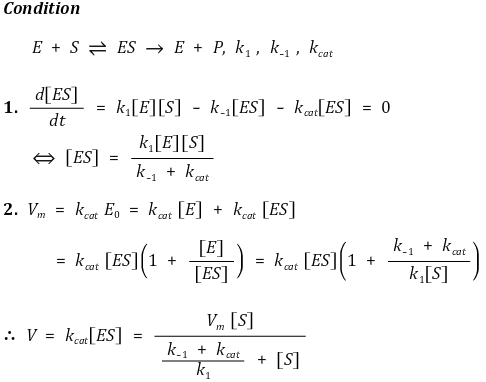
89. The mechanism shown below has been proposed for the enzyme-catalyzed hydrolysis of certain biochemical compounds (substrates), where ES is an enzyme-substrate complex. Given a fixed amound of enzyme, E, which of the following could be the plot of the initial rate of the production of product, P, when using varying initial concenctration of substrate, [S]0?
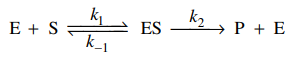
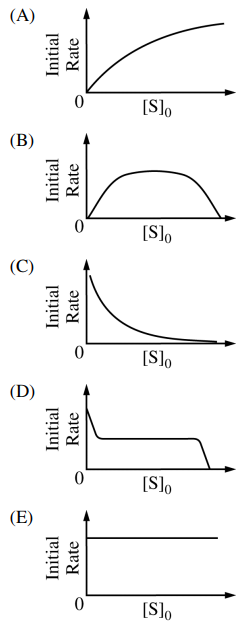
⑴ answer: A
90. The rate constant of a bimolecular gas phase reaction is found to follow the Arrhenius equation shown below. Which of the following will result in a smaller rate constant?
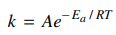

⑴ answer: B
91. If the dissociation of X2 proceeds by the elementary process shown below, the rate of change in [X] with respect to time is given by
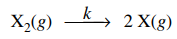
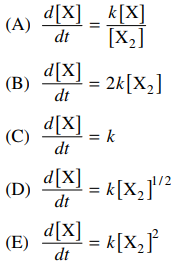
⑴ answer: B
⑵ the concept of elementary process
92. The compound shown below is AZT, a drug used in the treatment of acquired immune deficiency syndrome (AIDS). What is the total number of stereoisomers for this compound?
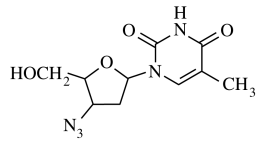
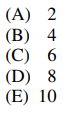
⑴ answer: D
⑵ The number of stereogenic centers is 3, thus the number of stereoisomers is 23 = 8.
⑶ nitrogen itself should be neglected by nitrogen inversion
93. What is the stereochemistry of the carbohydrate structure shown below?

⑴ anwser: D
⑶ ► RS nomenclature and cross-product
94. The enzyme-catalyzed transformation below, which occurs in the citric acid cycle (tricarboxylic acid or Krebs cycle), is best described as belonging to which of the following categories of reactions?

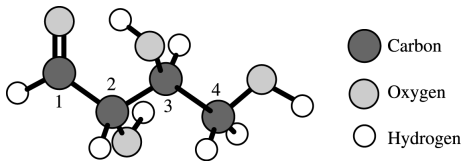
⑴ answer: A
95. Which of the following is NOT true about the disaccharide lactose shown below?
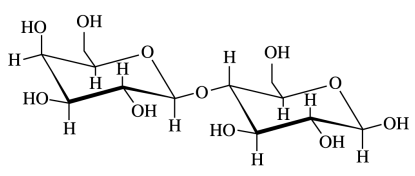
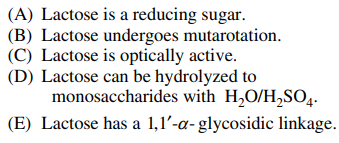
⑴ answer: E
⑵ A - Only sugar is not a reducing sugar among disaccharides.
⑶ B - Mutarotation is the change in the optical rotation because of the change in the equilibrium.
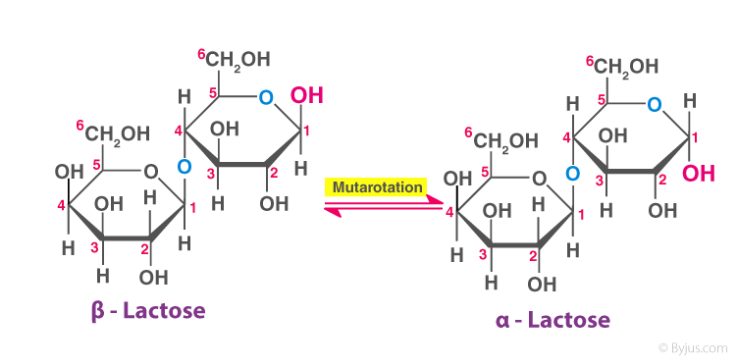
Figure. 1. mutarotation of lactose
⑷ C - It is noted that the left part of monosaccharide and the right part of monosaccharide differ.
⑸ D - Ether can be hydrolyzed by strong acids. (∵ reverse reaction of ether synthesis)
⑹ E - Lactose has a 1,4-β-glycosidic linkage. plus, the carbon just to the right of oxygen in the hexagonal ring is No. 1 carbon.
96. The compound shown below is a
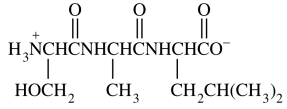

⑴ answer: C
⑵ What we need to do is counting C=O bonds or substituents.
97. A peptide digest yields the three polypeptides listed below. The three peptides are separated using capillary electrophoresis at a pH (above 3) at which each peptide has the same total positive charge. Which of the following indicates the order, from first to last, that the peptides will reach the detector? (A = alanine; L = leucine; G = glycine; K = lysine).
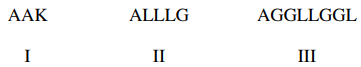
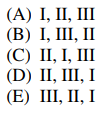
⑴ answer: A
⑵ Among the given peptides, only K (lysine) is positive charged amino acid.
⑶ The smaller, the faster in electrophoresis.
98. In fluorescence spectroscopy, the quantum yield (Φf) is best defined as the
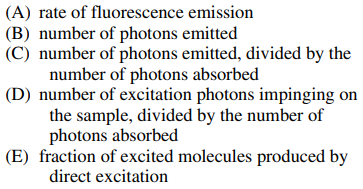
⑴ answer: C
99. When ferric oxide, Fe2O3, is dissolved in 6 M HNO3, which iron-containing species predominates in solution?
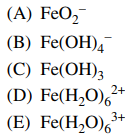
⑴ answer: E
⑵ A - Oxygen doesn't come into as a ligand for Fe.
⑶ B, C - In the acidic condition, OH- doesn't come into as a ligand.
⑷ D - In acid-base reaction, the given oxidation number of Fe, +3, doesn't change.
100. Of the following ionic substances, which has the greatest lattice enthalpy?
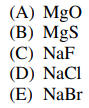
⑴ answer: A
⑵ the interionic energy is as follows
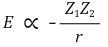
입력: 2022.03.05 01:12
'▶ 자연과학' 카테고리의 다른 글
| 【GRE】 Solution for GRE Chemistry Practice Test (0) | 2022.03.09 |
|---|---|
| 【GR1727】 The Solution for GRE Chemistry Practice Test [101-130] (0) | 2022.03.05 |
| 【GR1727】 The Solution for GRE Chemistry Practice Test [61-80] (0) | 2022.03.04 |
| 【GR1727】 The Solution for GRE Chemistry Practice Test [41-60] (0) | 2022.03.03 |
| 【GR1727】 The Solution for GRE Chemistry Practice Test [21-40] (0) | 2022.03.01 |




최근댓글