The Solution for GRE Chemistry Practice Test [101-130]
추천글 : 【GRE】 Solution for GRE Chemistry Practice Test
101. Which of the following reactions is best classified as an oxidative addition?

⑴ answer: D
⑵ Oxidation number of H in HCl is +1 (oxidized).
⑶ Oxidation number of H in [Pt{P(C2H5)3}2(H)2Cl2] is 0 (reduced).
⑷ Simultaneously, the oxidation number of Pt increases by 1 (oxidized).
102. Of the following colligative properties, which is most practical for determining the extent of protein aggregation?

⑴ answer: A
⑵ When protein aggregates, the molar concentration of solvents decreases; thus, osmotic pressure decreases.
103. A set of hybrid sp3 orbitals for a carbon atom is given below. Which of the following is NOT true about the orbitals?


⑴ answer: E
⑵ The explanation is based on valence band theory.
⑶ Each hybrid orbital may hold 2 electrons. (∵ Hund's rule)
104. According to Hückel molecular orbital theory, the secular equation below can be used to find possible energy levels of the π-electrons in
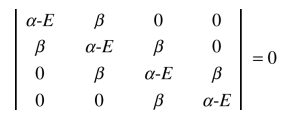
⑴ answer: D
⑵ The matrix depicts 4 positions in a row.
105. Which of the following is(are) characteristic of mass spectrometry?
Ⅰ. Analyte molecules are converted to gaseous ions.
Ⅱ. The ions are separated according to their mass-to-charge ratio.
Ⅲ. In addition to compound identification, mass spectra can be utilized to determine precise isotopic masses and isotopic ratios.

⑴ answer: E
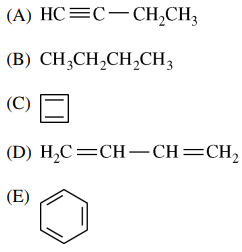
106. Which of the following is the major rearrangement product of the reaction shown below?
⑴ answer: C
⑵ SN1 reaction with ring opening reaction by carbocation rearrangement
107. Which of the following substituents is NOT an ortho, para director in an electrophilic aromatic substitution reaction?
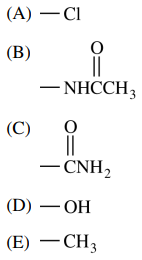
⑴ answer: C
⑵ Partial positive charge on carbonyl carbon makes the substituent as electron withdrawing group (EWG).
108. The reaction of terephthaloyl chloride with ethylene glycol, shown below, foms a
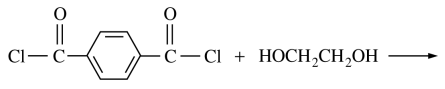

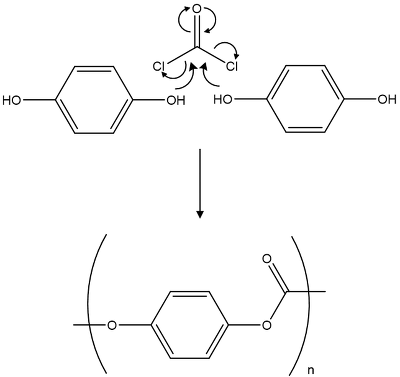
⑴ answer: B
⑵ Another example of polyester synthesis is as follows.
109. The proton NMR spectrum of an aromatic compound, C8H8Br2, includes two methyl singlets. Its proton-decoupled 13C NMR spectrum displays a total of six peaks. Of the following, which structure best fits these data?
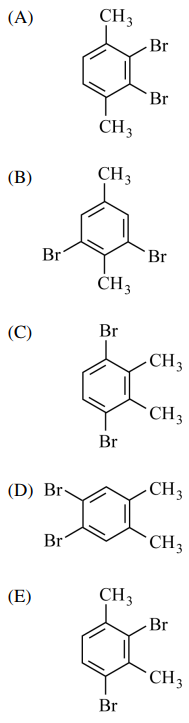
⑴ answer: B
⑵ NMR theory is as follows
⑶ A - 1 methyl singlet, 4 carbon peaks
⑷ B - 2 methyl singlets, 6 carbon peaks
⑸ C - 1 methyl singlet, 4 carbon peaks
⑹ D - 1 methyl singlet, 4 carbon peaks
⑺ E - 2 methyl singlets, 8 carbon peaks
110. The fact that infrared absorption frequency of deuterium chloride (DCl) is shifted from that of hydrogen chloride (HCl) is due to the differences in their

⑴ answer: E
⑵ Deuterium and hydrogen differ only in mass.
111. In the vibrational-rotational spectrum of a diatomic molecule, the R-branch of the spectrum is the result of which of the following transitions?
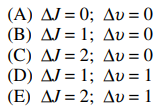
⑴ answer: D
112. When an activated complex is formed from two reactant molecules in the gas phase, it is usually assumed that the entropy has been lowered; that is, ΔS‡ is less than zero. This assumption is based on which of the following?

⑴ answer: E
113. A student performs five titrations and obtains a mean result of 0.110 M, with a standard deviation of 0.001 M. If the actual concentration of the titrated solution is 0.100 M, which of the following is true about the titration results?

⑴ answer: B
⑵ accuracy and precision
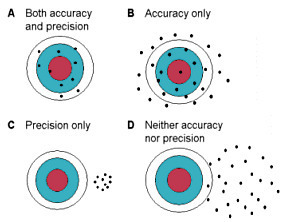
Figure. 1. accuracy and precision
114. Which of the following statements about the lanthanide elements is NOT true?
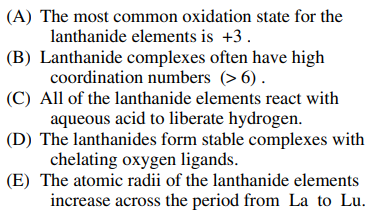
⑴ answer:E
⑵ No matter whether they are lanthanide elements or not, the atomic size decreases with atomic number across the period.
115. The equilibrium constant for the formation of Ni(en)32+, shown below, is 1010-fold greater than the equilibrium constant for the formation of Ni(NH3)62+. The primary explanation for this large difference is termed the
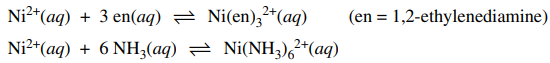

⑴ answer: E
116. Which of the following is a true statement about optical isomerism of complexes containing achiral ligands?
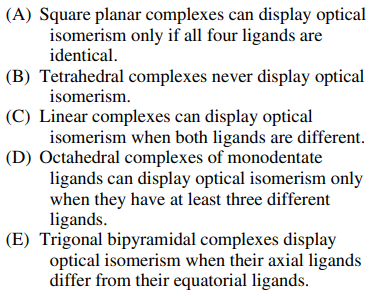
⑴ answer: D
⑵ optical isomers: Two compounds which contain the same number and kinds of atoms, and bonds (i.e., the connectivity between atoms is the same), and different spatial arrangements of the atoms, but which have non-superimposable mirror images.
⑶ A - If all ligands are identical, optical isomerism cannot be established.
⑷ B - Optical isomerism can be achieved when all ligands are different.
⑸ C - Linear complexes cannot display optical isomerism.
⑹ E - When the axial lignads are identical and the equatorial ligands are identical, optical isomerism cannot be achieved.
117. An organic compound has a distribution coefficient, Kp, of 2.00 between an ether and water. If 10.0 g of the compound is dissolved in 100 mL of water that is then extracted twice with 100 mL portions of the ether, what fraction of the compound remains in the water? (Kp = Cether / Cwater)

⑴ answer: A
⑵ For each extraction, 1/3 compounds can be remained since twice as many compounds are allocated to ether, when ether and water have the same volume.
⑶ The fraction of remained water = (1/3)2 = 0.111.
118. Exact solutions of the Schrödinger equation CANNOT be obtained for a

⑴ answer: E
⑶ particle in a one-dimensional box
119. When the Heisenberg uncertainty principle is applied to a quantum mechanical particle in the lowest energy level of a one-dimensional box, which of the following is true?

⑴ answer: E
⑵ Heisenberg uncertainty principle
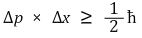
120. A reactant, R, can produce either of two products, P1 or P2, with competing pathways, as illustrated in the reaction profile shown below. If the reaction is carried out at low temperature, which of the following best indicates the preferred product and the type of control?
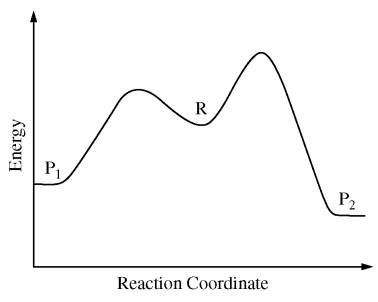
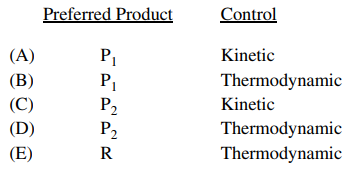
⑴ answer: A
121. For the titration reaction A + B → C, where A = analyte, B = titrant, and C = product, the end point is to be detected spectrophotometrically at 550 nm, based on the absorbance information shown below. The shape of the titration curve at 550 nm would most closely resemble which of the following?

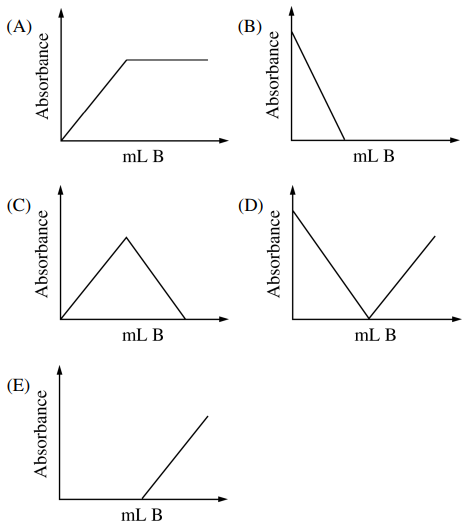
⑴ answer: D
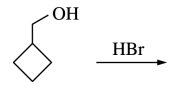
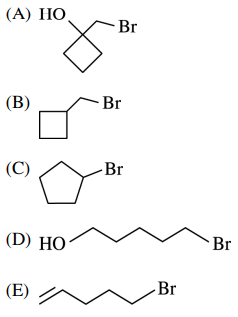
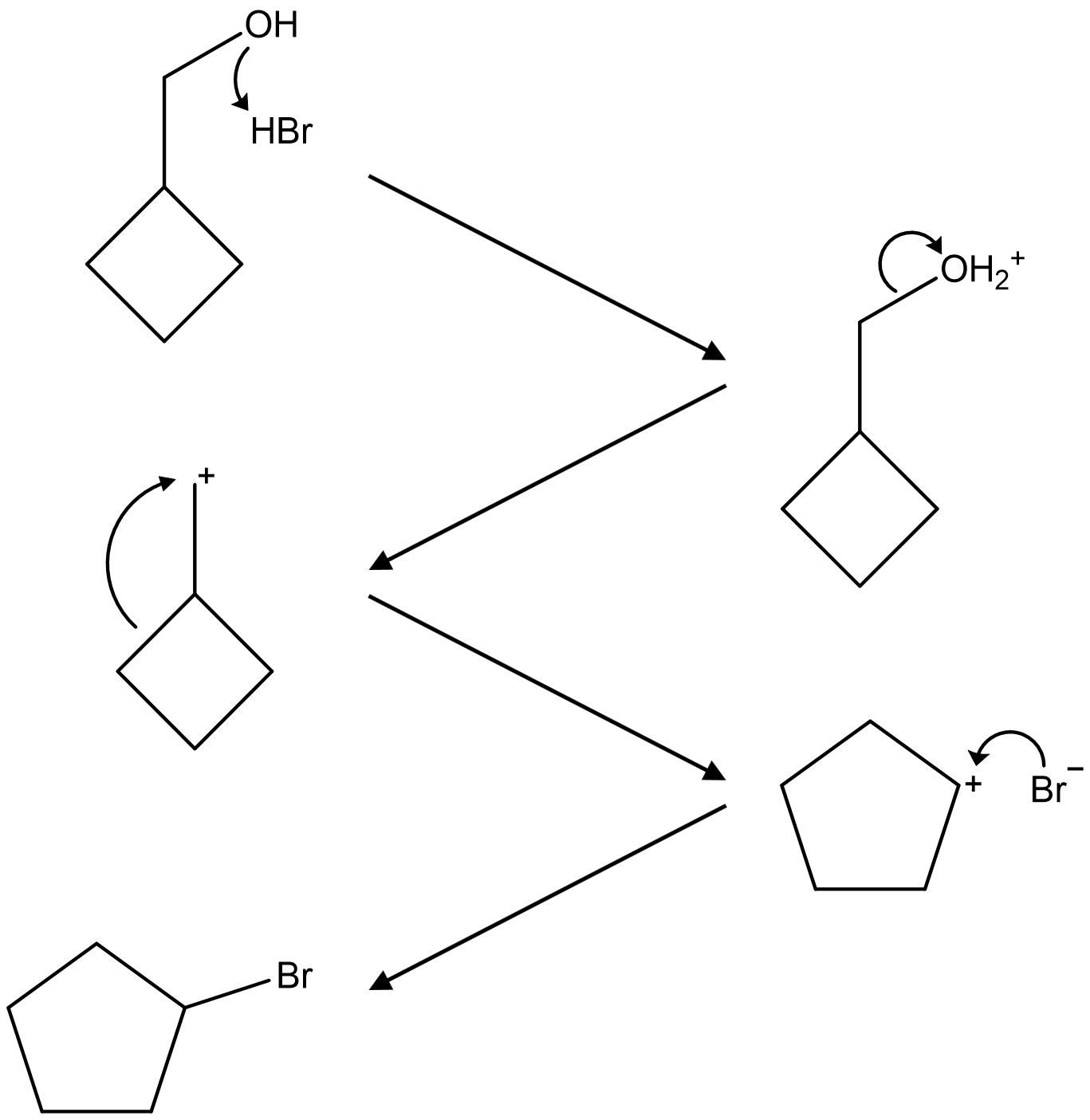
122. Which of the following reagents can be used to convert cyclopentanol to bromocyclopentane, as shown below?
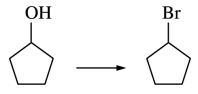
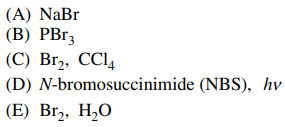
⑴ answer: B
123. Reduction of D-xylose with NaBH4 yields a product that is a
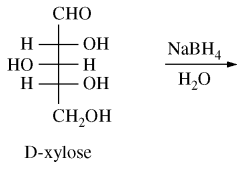

⑴ answer: E
⑶ meso compound : A compound that has more than 2 chiral centers but is not optically active due to inner symmetric plane .
124. Which of the following structures represents the amino acid lysine at pH 1?
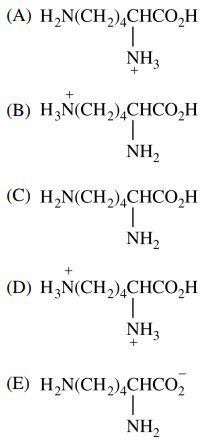
⑴ answer: D
⑵ pKa of carboxylic acid residue = 2.34
⑶ pKa of amino residue = 9.60
125. The reaction sequence shown below can be used to prepare benzocaine from 4-nitrotoluene. Which of the following reaction sequences would accomplish this synthesis?
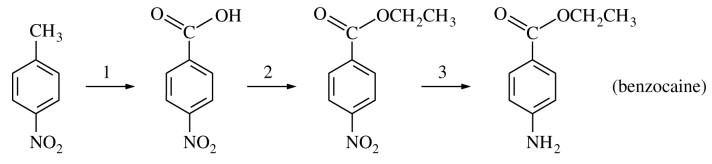
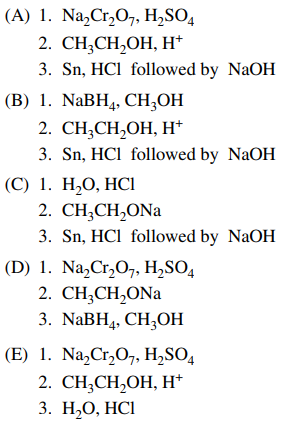
⑴ answer: A
⑵ step 1. benzyl carbon oxidation reaction
⑶ step 2. Fischer esterification
⑷ step 3. Clemmensen reduction
126. Which two of the following are the propagation steps in the free-radical chlorination of methane shown below?
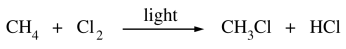
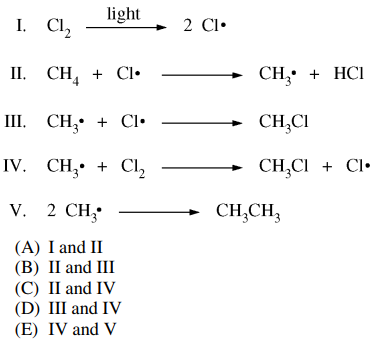
⑴ answer: C
⑵ Ⅰ : initiaton
⑶ Ⅱ : propagation
⑷ Ⅲ : termination
⑸ Ⅳ : propagation
⑹ Ⅴ : termination
127. What is the limiting high-temperature molar heat capacity at constant volume (CV) of a gas-phase diatomic molecule?
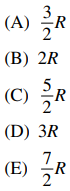
⑴ answer: E
⑵ I think the answer is C (reference)
128.The reaction energy diagram for the electrophilic bromination of benzene with Br2 and FeBr3 is shown below. Which position on the diagram corresponds to the species shown below?
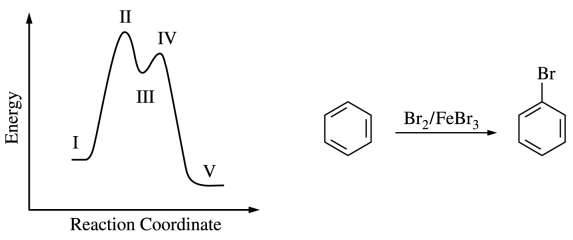
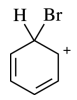
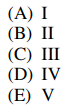
⑴ answer: C
⑵ Benzene halogenation is one of electrophilic aromatic substitution reaction (EAS).
129. Of the following atoms, which has the lowest electron affinity?
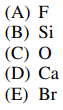
⑴ answer: D
⑵ electron affinity : Refer to the fact that the chemical species with the highest electron affinity is Cl .
130. Which of the following is a primary standard for use in standardizing bases?

⑴ answer: D
입력: 2022.03.05 18:00
'▶ 자연과학' 카테고리의 다른 글
| 【자연과학】 탐구 주제 리스트 (41) | 2022.05.08 |
|---|---|
| 【GRE】 Solution for GRE Chemistry Practice Test (0) | 2022.03.09 |
| 【GR1727】 The Solution for GRE Chemistry Practice Test [81-100] (0) | 2022.03.05 |
| 【GR1727】 The Solution for GRE Chemistry Practice Test [61-80] (0) | 2022.03.04 |
| 【GR1727】 The Solution for GRE Chemistry Practice Test [41-60] (0) | 2022.03.03 |



최근댓글